What's Matter? All in your environment is made up of matter. Atoms and substances are made up of minuscule pieces of matter. The atoms that make up the objects you see and touch every day are made up of these atoms. All that has mass and occupies space is referred to as matter (it has volume).
What exactly is Mass? The amount of matter in an object is measured by its mass. You may have a small object with a large mass, such as a lead statue (Pb). You might have a large object with a small mass, such as a helium-filled balloon (He). It's also important to understand the distinction between mass and weight. Weight is a measure of gravity's pull on an object, while mass is a measure of matter in an object.
What is Volume? The volume of something is the amount of space it takes up. Volumes are defined using terms like large, small, long, and short. A marble takes up a small amount of space, while a star takes up a lot of space. Various states of matter can fill volumes in various ways.
Even though matter exists anywhere in the Universe, it only exists in a few forms (states) on Earth. On the web, we cover five different states of matter. A phase is a term used to describe each of these states. In extreme conditions, a variety of other states of matter exist. If we continue to study the Universe, scientists will most likely discover more states of matter.

A state of matter is one of the distinct different forms that matter takes. In ordinary routine, four states of matter: solid, liquid, gas, and plasma are observable. Many other states like neutron-degenerate matter, Bose-Einstein condensates are known, but only in extreme conditions like ultra-cold or ultra-dense matter. Other states are supposed to be probable, such as quark-gluon plasmas, but for now theoretical.
The differentiation has traditionally been made based on qualitative variations in properties. The volume and form of matter in the solid state are constant, with component particles (atoms, molecules, or ions) close together and set in place. The amount of matter in the liquid state remains constant, but its form changes to match the container. Its particles are still close together, but they are free to move about. The volume and shape of gaseous matter are both variables, and it adapts both to suit its container. Its particles are not set in place nor close together. The volume and form of matter in the plasma state are variable, but it includes a large number of ions and electrons, all of which can freely pass about. The most abundant source of visible matter in the universe is plasma.
The four fundamental states
Solid

The particles (ions, electrons, or molecules) in a solid are tightly packed together. Since the forces between particles are so strong, the particles can only vibrate rather than move freely. As a consequence, a solid has a definite volume and a stable, definite form. Solids may only change their shape by applying force to them, such as when they are broken or cut.
The particles (atoms, molecules, or ions) in crystalline solids are packed in regular order, repeating patterns. Different crystal structures exist, and the same material may have several structures (or solid phase). At temperatures below 912 °C, iron, for example, has a body-centered cubic structure, whereas between 912 and 1394 °C, it has a face-centered cubic structure. At different temperatures and pressures, ice has fifteen different crystal structures or solid phases.
Since glasses and other non-crystalline, amorphous solids with no long-range order are not thermal equilibrium ground states, they are classified as nonclassical states of matter.
Solids can be melted to become liquids, and they can also be sublimated to become gases.
Liquid

A single atom liquid with a classic structure. Even though atoms have several nearest neighbors in contact, there is no long-range order.
A liquid is a nearly incompressible fluid that conforms to its container's shape while retaining a (nearly) constant volume independent of pressure. If the temperature and pressure remain constant, the volume is fixed. When a solid is heated past its melting point and the pressure is greater than the substance's triple point, it becomes liquid. While intermolecular (or interatomic or interionic) forces still exist, the molecules have enough energy to move about and the structure is mobile. This implies that the form of a liquid is dictated by its container rather than the liquid itself. The volume is normally greater than that of the equivalent solid, with water (H2O) being the most well-known exception. The critical temperature of a liquid is the maximum temperature at which it can live.
Gas

Gas molecules have a lot of space between them. The bonds between gas molecules are either very weak or non-existent. The molecules in "gas" are able to travel easily and quickly.
Gas is a substance that can be compressed. Gas will not only adapt to the shape of the container it is in, but it will also expand to fill it.
The molecules in a gas have enough kinetic energy that intermolecular forces have a small effect (or none at all in an ideal gas), and the average distance between adjacent molecules is much larger than the molecular dimension. A gas has no distinct form or thickness, but it takes up the entire volume of the container in which it is contained. A liquid may be converted to a gas by heating it to boiling point at constant pressure, or by lowering the pressure at a constant temperature.
A vapor is a gas that can be liquefied by compression alone without cooling at temperatures below its critical temperature. When vapor and a liquid (or solid) are in equilibrium, the gas pressure equals the vapor pressure of the liquid (or solid).
A supercritical fluid (SCF) is a gas with a temperature and pressure that are both higher than the critical temperature and pressure. The difference between liquid and gas vanishes in this condition. The physical properties of a supercritical fluid are similar to those of a gas, but its high density confers liquid properties in some situations, allowing for useful applications. In the production of decaffeinated coffee, for example, supercritical carbon dioxide is used to extract caffeine.
Plasma
Electrons are separated from their nuclei in a plasma, creating an electron "sea." It has the capacity to conduct electricity as a result of this.
Plasma, it's like gas has no specified form or volume. unlike gases, Plasmas are electrically conductive, generate magnetic fields and electric currents, and are sensitive to electromagnetic forces. Positively charged nuclei float in a "sea" of freely moving disassociated electrons, much as they do in conductive metal. In reality, it is this electron "sea" that enables plasma matter to conduct electricity.
The plasma state is often misunderstood, but it is very normal on Earth, and most people see it daily without even realizing it. Electric sparks, fluorescent lights, neon lights, plasma televisions, certain forms of flame, and stars are all examples of illuminated matter in the plasma state.
Gas is typically converted to plasma in one of two ways: by exposing it to extremely high temperatures or by creating a large voltage gap between two points.
As the matter is heated to high temperatures, electrons exit the atoms and are released, leading to the presence of free electrons. It is thought that at extremely high temperatures, such as those found in stars, virtually all electrons are “free,” and that a very high-energy plasma is simply bare nuclei floating in a sea of electrons.
Changing states of Matter
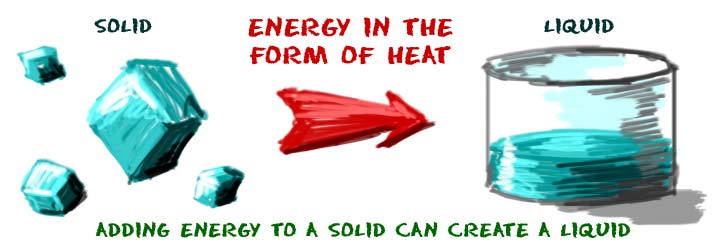
How does matter change from state to state? When certain physical conditions change, elements and compounds can move from one state to another. For instance, when the system temperature rises, the matter is excited and active in the system. A phase change can occur as the matter moves into a more active state when enough energy is put into a system.
Say you've got a glass of water (H2O). When the water temperature rises, the molecules become much more excited and rebound. It escapes from the liquid and becomes a gas if you give a liquid water molecule enough energy. The extra energy permits a change in the molecules.
"Phase" describes matter's physical condition. It's about 'Physical'. Matter moves only through physical means from one stage to another. If energy is added (temperature is increased) or if energy is removed (something freezes), physical change can be created.

Another way to make a physical change is to change the pressure in a system. A glass of liquid water will simply sit on a table. You can watch the water boil and the water molecules move to a gas phase if you place a glass of water in a vacuum chamber and reduce the pressure.
Molecules remain the same substance when they transform from one phase to the next. Water vapor rises from a pot of boiling water. In the cooler air, that vapor (or gas) can condense and become a drop of liquid water. If you put that liquid drop in the freezer, it will solidify into a block of ice. It was always water, regardless of its physical state. Even though the physical state had changed, the chemical properties remained unchanged.
A chemical change, on the other hand, would strengthen or weaken the chemical bonds in the water (H2O) molecules. Formaldehyde is created by adding a carbon (C) atom (H2CO). You can make hydrogen peroxide by adding an oxygen (O) atom (H2O2). Neither new compound resembles the original water molecule in any way. In most cases, changes in the physical state of a compound do not result in a chemical change.

All matter has the ability to change states. It may necessitate extreme temperatures or pressures, but it is possible. A substance may refuse to change states at times. When that happens, you'll have to use all of your tricks. To make a solid, you may need to lower the temperature greatly and then add pressure. At standard pressure, oxygen (O2) solidifies at -361.8 degrees Fahrenheit (-218.8 degrees Celsius). When the pressure is increased, it will freeze at warmer temperatures.
Liquid nitrogen (N2) is something that some of you may be familiar with. It's liquid nitrogen from the atmosphere, and it has to be extremely cold to stay liquid. What if you wanted to solidify it but couldn't get it cold enough to do so? In a sealed chamber, you could raise the pressure. You'd eventually reach a point where the liquid solidified. If you have liquid water (H2O) at room temperature and want to make water vapor (gas), you could use a combination of high temperatures and low pressures.
When matter hits certain points, the phase will change. A liquid has the desire to solidify at times. The temperature at which a liquid transforms into a solid is measured by a freezing point or melting point, as described by scientists. The melting point can be affected by specific physical factors. one of those factors is pressure. The freezing point and other particular points of a material rise as the pressure around it rise. When things are under more pressure, it's easier to keep them solid.
Solids are generally denser than liquids due to the closer spacing of their molecules. The molecules are compressed into a smaller area during the freezing process.
In science, there are always exceptions. Water is special in several ways. When it is frozen, there is more distance between the molecules. When the molecules are all loosey-goosey in the liquid state, they assemble in a complex structure that takes up more room. Solid water is less dense than liquid water so the same amount of molecules take up more volume. In solid water, there are far more forms of molecular organizations than we can discuss here.
CHEMISTRY TERM PHASE CHANGE
Fusion/Melting Solid to a Liquid
Freezing Liquid to a Solid
Vaporization/Boiling Liquid to a Gas
Condensation Gas to a Liquid
Sublimation Solid to a Gas
Deposition Gas to a Solid
Solid to a Liquid < > Liquid to a Solid
Consider yourself a solid. The ice is on the counter, you are a piece of ice. You dream that you're becoming water liquid. You need energy. Heat is potentially the best energy to modify your physical condition. The liquid atoms have more power than the solid atoms.
Each substance has a melting point at a special temperature. If a solid exceeds its melting point temperature, it can become a liquid. To melt water you must have a temperature of a little more than 0 °C.
Your melting point would be greater than water if you were salt, sugar, or rock. How did you come to that conclusion? When the temperature is above zero degrees Celsius, they would be liquids if their melting points were lower. Freezing is the reversal of the melting mechanism. When the molecules of liquid water lose momentum, they freeze and solidify into ice.
Solid to a Gas < > Gas to a Solid

You've learned about solids melting and changing into liquids. Any of you may have seen the transformation of a solid into a gas. Sublimation is the term for the method. Dry ice is perhaps the clearest example of sublimation. Dry ice is a form of carbon dioxide (CO2) that is solid at room temperature. Dry ice, when left out in a room, magically transforms into a gas. If you know what liquid carbon dioxide is? It can be done, but not in ordinary situations. At natural atmospheric pressures, coal is another example of a compound that will not melt. At extremely high temperatures, it will sublimate.
Is it possible to transform from a gas to a solid? And, of course. When a gas becomes a solid without passing into the liquid state of matter, this is called deposition. Many of you who live near the equator may not have seen it, but frost can be observed on winter mornings closer to the poles. When the water vapor from the air freezes as a solid on the leaves of plants, those small frost crystals form.
Liquid to a Gas < > Gas to a Liquid
If you're a liquid and want to turn into a gas, you'll need a lot of energy. Your molecules will begin to vibrate until you can funnel the energy into them. They will break the confines of the liquid environment and become a gas if they vibrate enough. When the molecules in your system have enough capacity to form a gas, you've reached your boiling point.
If you're a gas, the opposite is true. Your very excited gas atoms need to lose some energy. The simple solution is to reduce the ambient temperature. As the temperature decreases, energy from the gas atoms is transferred to the cooler environment. You become a liquid when you exceed the temperature of the condensation point. If you were a water vapor floating over a boiling pot of water and hit a wall, the wall would be fine, absorbing some of your extra energy, and you'd easily transform into a liquid. Energy is also absorbed by cooler objects from hotter objects.
Gas to a Plasma < > Plasma to a Gas

Let's end by imagining yourself as a neon (Ne) gas. You say I'd like to become a plasma. You're halfway there as a gas, but you do need to rip a couple of electrons out of the atoms. Ionization of the gas is required. The charge of electrons is negative. Eventually, you'll have almost identical concentrations of positively and negatively charged particles. They're covered in a big plasma ball. The charge of the entire plasma is near to neutral when the positive and negative charges are in equal proportions. When a large number of positively charged particles cancel out the charges of an equivalent number of negatively charged particles, the result is neutral.
If a large amount of energy is pushed into gas, plasma can be formed. The electrons in neon are pulled off by electrical energy. Simply turn off the neon light switch when you're ready to become a gas again. The neon plasma reverts to a gaseous state when the atoms are not energized by electricity. Here on Earth, we have a unique world. There isn't a lot of plasma in our environment. Plasma is found everywhere once you leave Earth and travel through space. It's in the stars and everything between them.

It's essential to consider the difference between chemical and physical changes. Some changes are obvious, but there are some fundamental concepts to be aware of. Physical changes usually refer to changes in the physical state of matter. When two or more molecules interact, chemical changes occur on a molecular level. When atomic bonds are broken or formed during chemical reactions, a chemical reaction occurs.
Molecules Do Not change
You've forced a physical change when you step on a can and crush it. You only changed the shape of the can, though. Because the energy in the can did not change, it was not a change in the state of matter. Furthermore, because this was a physical change, the molecules in the can remain the same. There were no chemical bonds formed or broken. When you melt an ice cube (H2O), you add energy, which causes a physical change. You exerted sufficient energy to cause a phase change from solid to liquid. Physical changes can occur as a result of physical actions such as changing the temperature or pressure. When you melted the ice, no chemical changes occurred. Water molecules will always be water molecules.
Chemical transformations take place on a much smaller scale. While some experiments reveal visible chemical changes, such as a change in color, the majority of chemical changes are not visible. The chemical change as hydrogen peroxide (H2O2) becomes water is invisible, because both liquids are clear, However, billions of chemical bonds are formed and broken behind the scenes. You may notice oxygen (O2) gas bubbles in this example. The chemical changes are visible in the bubbles.
Because the substance is still sugar, melting a sugar cube is a physical change. A chemical change occurs when a sugar cube is burned. A chemical reaction between sugar and oxygen is triggered by the fire. The chemical bonds between the sugar and the oxygen in the air are broken.
When iron (Fe) is exposed to oxygen gas in the air, it rusts. Over a long time, you can observe the process. As the iron oxidizes, the molecules change the structure and eventually become iron oxide (Fe2O3). Real-world examples of the oxidation process include rusted pipes in abandoned buildings.
Some chemical changes are extremely small and take several steps to complete. The resulting compounds may have the same number of atoms, but their structure or atom combinations will be different.
Six carbon atoms, twelve hydrogen atoms, and six oxygen atoms (C6H12O6) make up the sugars glucose, galactose, and fructose. They are called isomers because, despite having the same atoms, they have very different shapes. Atoms are bonded in different orders in isomers.
Because of the differences in molecular structure, each sugar undergoes a different chemical reaction. According to scientists, the arrangement of atoms allows for a high degree of specificity, particularly in living things' molecules. The term "specificity" refers to the fact that the molecules will only work in certain reactions, not all of them. Your body, for example, uses glucose as an energy source. Before your body can use galactose molecules, they must first be converted to glucose.
Was this article helpful? Give it a share!




















No comments: